is co2 polar or nonpolar
Welcome to the Mentor Center. The reason the molecule is still nonpolar is because its geometry is linear and the polar bonds face in opposite directions.

Why Is Carbon Dioxide A Non Polar Molecule Physics Stack Exchange
The carbon-oxygen double bonds in the linear CO2 molecule are polar electronegativities.

. Thats the short answer regarding carbon dioxides non-polarity. Read the full answer. Is CO2 a covalent or ionic bond. What is polar and non-polar.
Polar molecules have a non-zero net dipole moment. And two O atom equally cancel the dipole moment of each otherso the net dipole moment is zero. Both has 2 atoms and share electrons with covalent bond. Question Is CH2 polar or nonpolar.
Is CO2 polar or nonpolar. Answer CH2 Methylene is Nonpolar What is polar and non-polar. All the charges are equally distributed and both the bond dipole moments are canceled. All fats and oils are non-polar thus using a non-polar solvent is most appropriate.
Answer COF2 Carbonyl fluoride is Polar. In this video we are going to answer this question. CO2 carbon dioxide is a nonpolar molecule because of its linear symmetric shape. Both CO2 and H2O have two polar bonds.
Carbon dioxide or CO2 for short is a greenhouse gas that contributes to global warming. As it is clear from the image that both electrons A and B attract electrons equally. However the dipoles in the linear CO2 molecule cancel each other out meaning that the CO2 molecule is non-polar. Carbon is often found in the form of carbon dioxide.
Answer 1 of 4. List molecules polar and non polar. Carbon dioxide CO2 is nonpolar due to its linear symmetrical structure. Because oxygen atoms make sigma bonds with the central carbon atom to complete their.
Why water is polar while Carbon dioxide is nonpolar. Carbon dioxide which has the chemical formula CO2 is non-polar. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Both CO2 and H2O have two polar bonds.
CO2 is a commonly kn. C 25 O 35. Even though they both has similarity. However it would be good to contextualize carbon dioxides non-polar attributes with.
What is the bond of CS2. CO2 is nonpolar in nature because there is no uneven sharing of valence electrons. Non-polar bonds share electrons equally between bonded atoms. Carbon dioxide CO2 is nonpolar although it contains polar bonds.
If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. However the dipoles in the linear CO2 molecule cancel each other out meaning that the CO2 molecule is non-polarThe polar bonds in the bent H2O molecule result in a net dipole moment so H2O is polar. As co2 has linear structure. Hence as there is no net molecular dipole moment in the molecules CO2 is a nonpolar molecule.
Since carbon and oxygen have different electronegativities the electrons are not shared equally between the two atoms. The two oxygen atoms in either direction of the carbon atom pull the electron density equally from both sides. The electrons in each of the double bonds are drawn toward the oxygens so both oxygen atoms have a partial negative charge. Examples of non-polar bonded molecules.
1 carbon atom 2 oxygen atom. Some common examples of non-Polar molecules are H2 Cl2 BeCl2 CO2 C2H2 BF3 CCl4 are some examples of nonpolar molecules. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Therefore it would be good to contextualize carbon dioxides non-polar attributes with water polar molecules and to go into detail about how a molecules polarity is decided.
CO2 carbon dioxide is Nonpolar. Although the carbon and oxygen differ in their electronegativity due to which CO bond is polar the polarity of both opposite CO bonds get canceled by each other due to symmetrical shape and result in a nonpolar molecule with zero dipole moment. Water is at the opposit side while Ethanol Methanol and Acetone in the middle not very unpolar not. It is non polar.
Is CO2 polar or nonpolar. Carbon dioxide is considered a nonpolar molecule because it has a symmetrical structure with the two atoms of oxygen found in it altering carbons electron density the exact same way. CO2 is made up of. It contains two polar bonds that are arranged symmetrically.
Non-polar compounds cannot dissolve in water so they require other solvents like hexane to break down into smaller pieces before they can be dissolved. When it comes to Carbon Dioxide it has a linear geometry as both the Oxygen atoms share double bonds with the central Carbon atom. Carbon and oxygen are non-metals thus we know carbon dioxide is a covalent compound. Dipole moment is determined from structure.
Carbon forms a double bond with each oxygen atom. Ill tell you the polar or nonpolar list below. In this video I find out whether carbon dioxide is polar or nonpolarElectronegativity chart. To answer the question Co2 polar or nonpolar we need to understand the molecular geometry of CO2In CO2 molecular geometry Carbon makes a double bond with each of the two oxygen atoms resulting in a tiny symmetrical linear molecule of CO2 that is volatile and reasonably reactive.
Meaning a non-polar solvent will dissolve a non-polar chemical. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. We are going to determine the molecular polarity for CO2. CO2 like Pentane and Hexane is very unpolar therefore the best solvent for oils and fats.
In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Is CO2 double bonded.
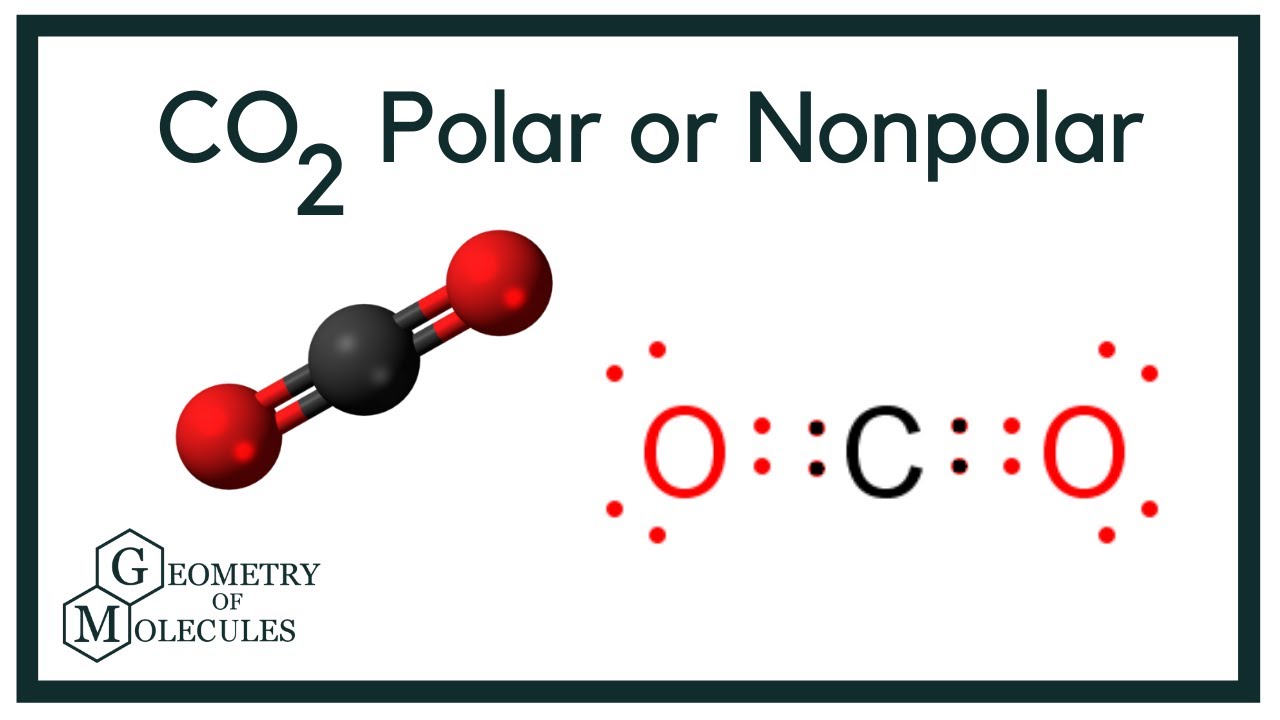
Is Co2 Polar Or Nonpolar Carbon Dioxide Youtube

Co2 Co2 Molecule Polar Or Nonpolar 1344x627 Png Download Pngkit
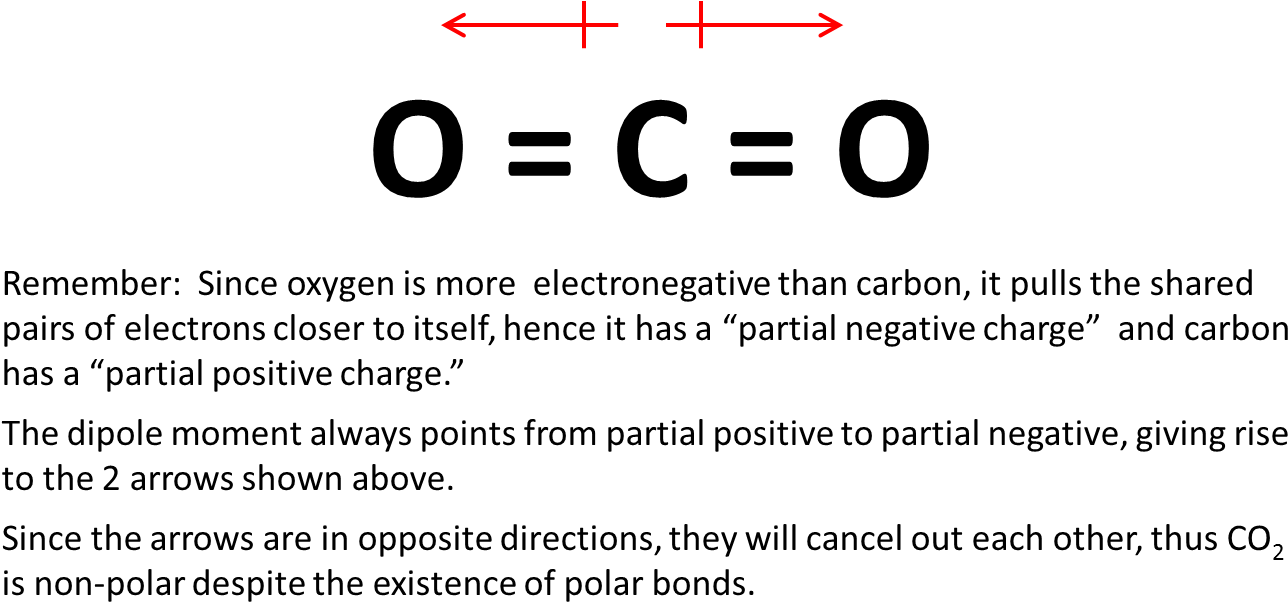
Download Co2 Co2 Molecule Polar Or Nonpolar Full Size Png Image Pngkit

Polar And Non Polar October 2013
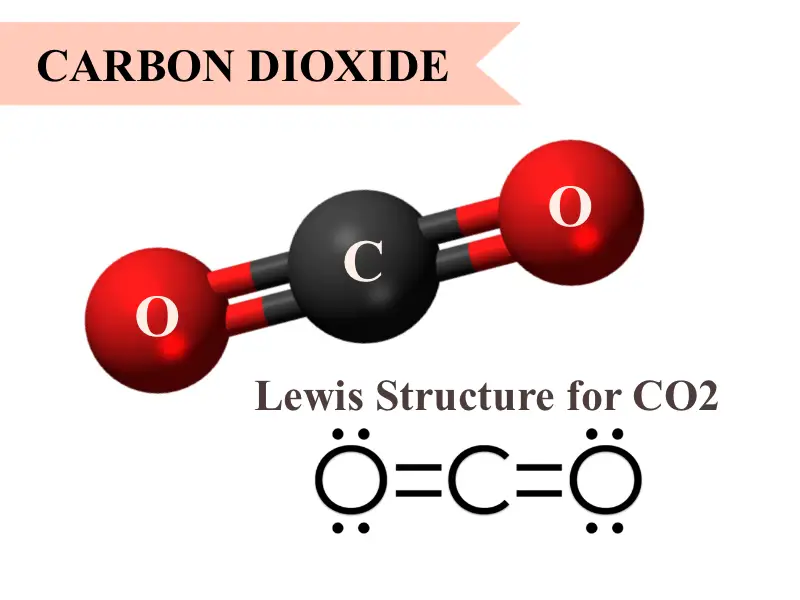
Is Carbon Dioxide Co2 Polar Or Nonpolar You Ask We Answer
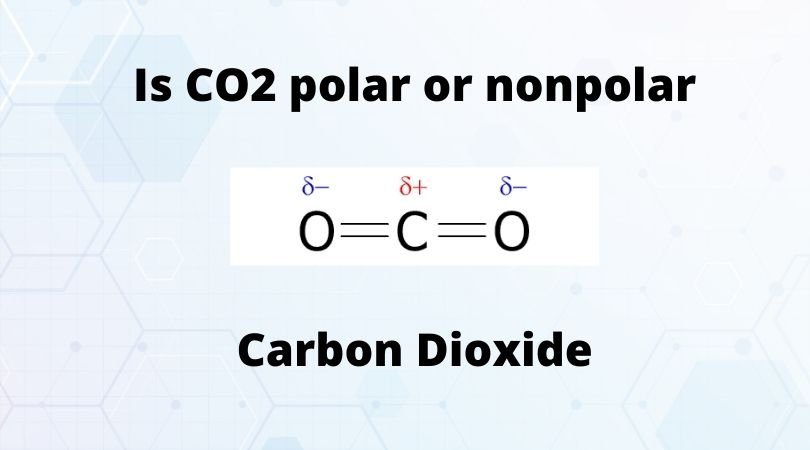
Is Co2 Polar Or Nonpolar Check Carbon Dioxide Polarity Geometry Of Molecules
Posting Komentar untuk "is co2 polar or nonpolar"